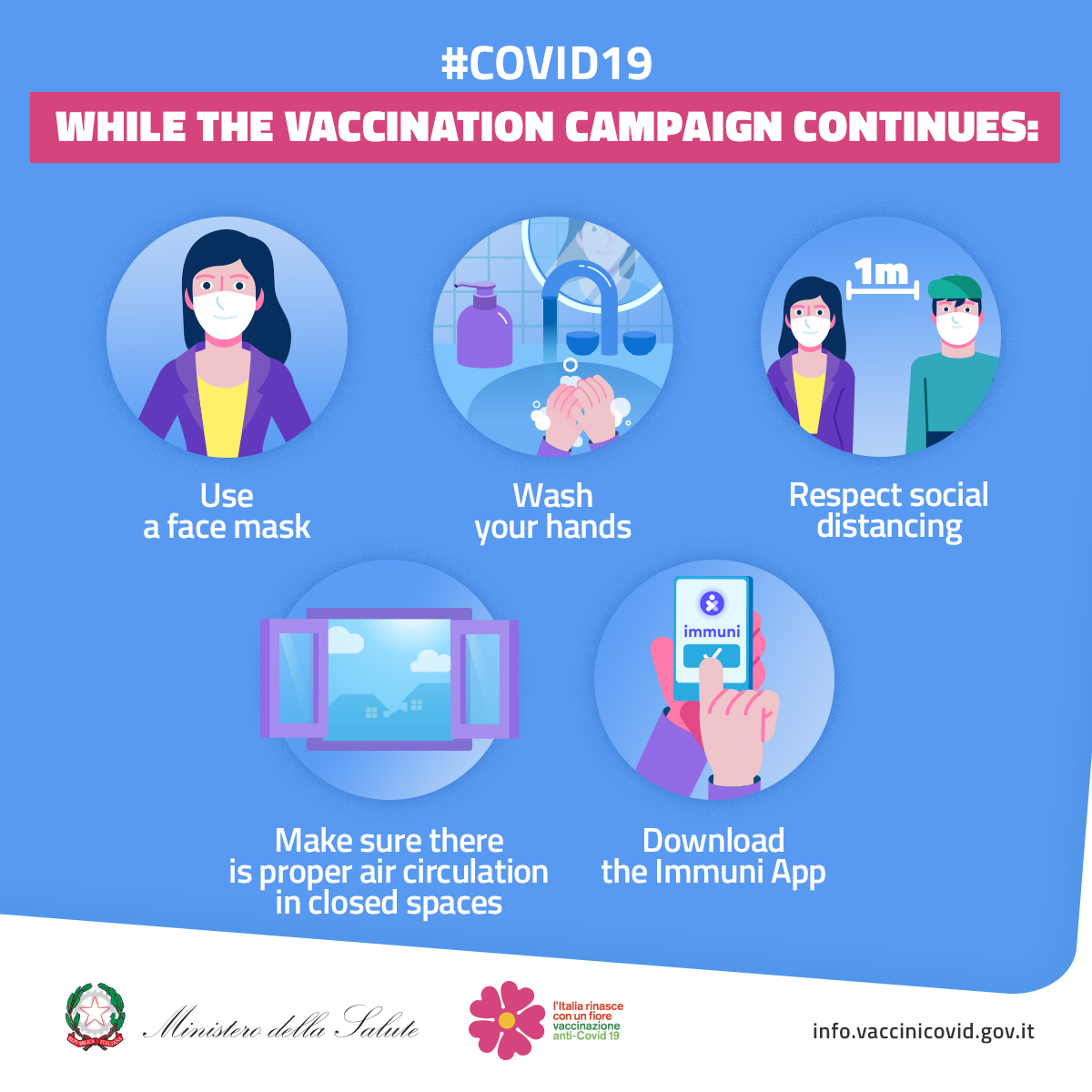
The recommendation issued by the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) during a meeting held on March 18, 2021 confirmed the favourable benefit/risk ratio of AstraZeneca's Covid-19 vaccine, ruling out any association between cases of thrombosis and the Covid 19 vaccine. It also ruled out quality and production issues, based on the available data.
After consultation with the Minister of Health, the Directorate-General for Prevention of the Ministry of Health, and the Consiglio Superiore di Sanità (CSS), AIFA announced that the reasons for the precautionary ban on a few vaccine batches, distributed on March 15, 2021, no longer applies.
Therefore, as soon as the Committee for Medicinal Products for Human Use (CHMP) issues its opinion, AIFA will proceed to lift the ban on the use of AstraZeneca vaccine, allowing the vaccination campaign to be fully resumed from today, Friday March 19, at 3pm.
Please refer to:
Consulta
le
notizie di Covid-19
Vai all' archivio completo delle notizie
Consulta l'area tematica:
Covid-19